Allergic contact dermatitis to nickel: Modified in vitro test protocols for better detection of allergen-specific response
R. Spiewak1,2, H. Moed3, B. M. E. von Blomberg4, D. P. Bruynzeel3, R. J. Scheper4, S. Gibbs3, T. Rustemeyer3
1Institute of Dermatology, Krakow, Poland, 2Celimun Biomedical Research, Krakow, Poland, 3Department of Dermatology, VU University Medical Centre, Amsterdam, the Netherlands, 4Department of Pathology, VU University Medical Centre, Amsterdam, the Netherlands
Source: Spiewak R, Moed H, von Blomberg BME, Bruynzeel DP, Scheper RJ, Gibbs S, Rustemeyer T. Allergic contact dermatitis to nickel: Modified in vitro test protocols for better detection of allergen-specific response. Contact Dermatitis 2007, 56 (2): 63-69.
|
Abstract: To date, no in vitro test is suitable for routine diagnosis of contact allergy. The aim of our study was to establish improved in vitro test protocol for the detection of antigen-specific responses of lymphocytes from patients with allergic contact dermatitis to nickel (Ni-ACD). Blood leucocytes from 14 Ni-ACD patients and 14 controls were cultured in the presence of 'cytokine cocktails' skewing lymphocytes towards 'type 1' [interferon-gamma (IFN-γ)-secreting] or 'type 2' [interleukin (IL)-5 and IL-13-secreting] phenotypes. The cocktails consisted of IL-7 and, respectively, either IL-12 or IL-4. Cell responses to nickel were measured with enzyme-linked immunospot assay (ELISpot), enzyme-linked immunosorbent assay (ELISA), and lymphocyte proliferation test (LPT). Significant differences between patients with Ni-ACD and controls were found for the 'type 2' cytokines IL-13 and IL-5, with further increase of allergen-specific responses occurring when cultures were supplemented with IL-7 and IL-4. No significant differences were found for IFN-γ. The best correlate to clinical diagnosis was LPT with 'type 2' skewing (r = 0.739, P < 0.001), followed by IL-13 ELISpot with 'type 2' skewing (r = 0.654, P < 0.001). The non-radioactive method that correlated best with LPT was IL-2 ELISpot (r = 0.809, P < 0.001). Overall, we conclude that combining ELISpot assay with proposed modifications of culture conditions improves detection of specific lymphocyte responses in contact allergy to nickel.
Key words: allergic contact dermatitis, lymphocytes, phenotype skewing, cytokine cocktails, ELISpot, ELISA, lymphocyte proliferation test.
|
Reprint (PDF)
Links:
Institute of Dermatology, Krakow, Poland
Sensimun - biomedical research outsourcing
ELISpot - consulting, outsourcing, plate scanning
|
For more than a century, patch tests have been the method of choice for the diagnosis of allergic contact dermatitis (ACD). Though indispensable, they have, however, certain limitations, such as inter-observer variability (1), site-to-site variability (2), and test-to-test variability (3). Patch test results may be influenced by the time of reading (4, 5), quality of allergens used (6), ultraviolet irradiation (7), topical and oral steroids (8, 9). In some cases, excessive irritation of the skin makes the interpretation of patch tests difficult or impossible - a situation referred to as "angry back" or "excited skin syndrome" (10, 11). Taking the above limitations into account, a reliable in vitro test for contact allergy would be greatly appreciated.
In the past decades, various in vitro tests have been used for the detection of contact allergy, starting with macrophage migration inhibition test (12) and lymphocyte blastic transformation test (13). Lymphocyte proliferation test (LPT) was introduced in the 1970s (14) and has been used until today, however, mainly for experimental purposes. Later, the above-mentioned methods were followed by analyses of cytokine and chemokine secretion, surface cell markers and gene expression. Unfortunately, none of these methods have proven sufficient for diagnostic use, mainly due to poor sensitivity and/or specificity. Combinations of 2 or 3 different in vitro methods or parameters have been proposed to overcome this problem (15, 16), however, also this approach has not found its way into routine clinical applications.
In a previous study (17), we have demonstrated that skewing lymphocytes towards "type 1" (IFN-γ-secreting cells) and "type 2" (IL-5 and IL-13 secreting cells) could improve detection of nickel-specific T cell response in contact allergy. Observations were also published suggesting that enzyme-linked immunospot assay (ELISpot) could offer advantages in detection of allergen-specific responses than other in vitro assays (18-20). In the present study, we combined both above-mentioned approaches to see whether this could offer any progress to the present state of the art. The aim was to determine whether combining modified culture conditions with ELISpot assay could improve the detection of nickel-specific response in vitro, as compared to the previously described methods.
Patients and Methods
Combinations of particular culture conditions and in vitro assays are in this article referred to as "test protocols". The design of the study and data analysis were aimed at answering the 3 following questions: Q1 "In which test protocol the differences between Ni-ACD patients and Controls are most pronounced and significant?" and Q2 "In which test protocol the results correlate best with clinical diagnosis?". As LPT has probably been the most popular in vitro assay in contact allergy for past decades, the question Q3 was "Which non-radioactive test protocol produces results that correlate best with LPT?".
Study group
14 female patients with confirmed nickel-allergic contact dermatitis (Ni-ACD) and 14 female Controls were studied. The inclusion criteria for the Ni-ACD group were: clear history of metal dermatitis and an at least ++ patch test reaction to 5% NiSO4 pet. after 48, 72 and/or 144 h ("golden standard" for this study). The control group consisted of 7 eczema patients and 7 healthy volunteers, with no signs of metal intolerance and negative patch test to nickel. Three persons among Ni-ACD and 4 among Controls had positive history of atopic diseases. The age range was 20-59 (median 36.5) years in the Ni-ACD group and 25-62 (median 36.5) years among Controls. The participants gave informed consent, and the study was accepted by the local ethics committee.
Cell cultures
PBMC were separated from the participants' blood samples in Ficoll-Paque Plus (Amersham, Uppsala, S) and cryopreserved in liquid nitrogen until testing. The cells were cultured in IMDM medium with penicillin, streptomycin and 1,4-dithiothreitol at 37°C, 95% RH, 5% CO2. The final cell density was 104 cells/well for IFN-γ ELISpot and 2×105 cells/well for all remaining ELISpot, ELISA and LPT assays. The cells were cultured in triplicate both with and without the presence of NiS04 (50 µM), and both with and without the addition of IL-7 and IL-4 or IL-12, referred to as "cytokine cocktails". The selection of nickel concentration was based on study results by Lindemann et al. (19), who compared seven different concentrations of nickel sulphate (from 6 to 200 µM) and found 50 µM most suitable for cell cultures, in terms of specific lymphocyte stimulation versus cytotoxicity. Two "cytokine cocktails" were used in the study: "7/4 cocktail" - a "type 2"-skewing combination of IL-7 and IL-4, and "7/12 cocktail" - a "type 1"-skewing combination of IL-7 and IL-12. All cytokines used were human recombinant proteins (Strathmann Biotec, Hannover, D) with specific activities of 5 × 107 units/mg for IL-7, 2 × 107 units/mg for IL-4, and 1 × 107 units/mg for IL-12. Final concentrations of the cytokines in cell cultures were 240 units ml-1 IL-4, 0.5 units ml-1 IL-7, and 10 units ml-1 IL-12. These concentrations were established in a series of introductory tests and proved effective in preceding studies done in our group (17, 21). A similar approach was also used by Jennes et al. for improving detection of virus-specific lymphocytes through culturing PBMC with IL-7 and IL-15 (22).
Enzyme-Linked Immunospot Assay (ELISpot)
The cells were cultured initially for 20 h in round-bottom 96-well culture clusters in order to enable good antigen presentation. Subsequently, the cells were moved to 96-well PVDF microfilter plates (Millipore, Molsheim, F), each coated with respective antibodies. After additional 40 h of culture for IFN-γ, and 5 days for IL-2, IL-5 and IL-13, the ELISpot assay was done following the manufacturers' guidelines. Antibodies for IFN-γ, IL-5, IL-13 ELISpot and streptavidin-alkaline phosphatase (s-ALP) conjugate were from Mabtech (Näcka, S), antibodies for IL-2 ELISpot were from R&D (Minneapolis, MN), the BCIP/NBT colour AP substrate was from Bio-Rad (Hercules, CA). Spots were counted automatically using the AID ELISpot Reader (AID, Straβberg, D).
Enzyme-Linked Immunosorbent Assay (ELISA) and Lymphocyte Proliferation Assay (LPT)
Cultures for ELISA and LPT were grown in round-bottom 96-well culture clusters for 6 days. IFN-γ in supernatant was measured with antibody pairs from Sanquin (Amsterdam, NL) and IL-5 with antibodies from BD (San Diego, CA) according to the manufacturers' recommendations. Colour reaction was developed with horseradish peroxidase (s-HRP) polymer (Sanquin) with OPD/H2O2 substrate, and measured with Microplate Autoreader EL311 (Bio-Tek, Winooski, VT). 3H-thymidine (Amersham, Little Chalfont, UK) was added to cell cultures for the last 5 h, after which the amount of incorporated radioactivity was measured using TopCount NXT from Packard Biosciences (Downers Grove, IL).
Data analysis
Altogether, results of 15 in vitro test protocols were compared: IL-2 ELISpot in cultures without and with the "7/12 cocktail", IL-5 ELISpot and ELISA without and with the "7/4 cocktail", IL-13 ELISpot without and with the "7/4 cocktail", IFN-γ ELISpot and ELISA without and with the "7/12 cocktail", and finally LPT without cocktail, with the "7/4 cocktail", and with the "7/12 cocktail". To answer question Q1 (influence of in vitro test protocols on measurable differences between Ni-ACD patients and Controls), test protocols which produced significant differences (P≤ 0.05, Mann-Whitney "U" test, 2-tailed), were ranked according to magnitude of differences between median results in Ni-ACD and Controls, expressed as per cent of the median result in Ni-ACD group. To answer question Q2 (concordance of in vitro results with clinical diagnosis), in vitro test protocols were ranked according to the coefficient of correlation (Pearson correlation, significance test 2-tailed) with the golden standard (positive history of nickel intolerance combined with at least a ++ patch test to Ni). To answer the question Q3 (concordance between non-radioactive test protocols and the LPT), non-radioactive test protocols were ranked from the highest to the lowest coefficient of correlation with LPT (Pearson, 2-tailed). Statistical package SPSS+ for Windows (SPSS Inc, Chicago, IL) was used for the above analyses.
RESULTS
Influence of protocol on differences between Ni-ACD patients and Controls (Q1)
In 6 of 15 in vitro protocols tested, differences between median results for Ni-ACD patients and Controls were larger than 90% and significant at P≤0.05 (Table 1). The differences of more than 100% in the case of IL-13 ELISpot with "7/4 cocktail" are due to the fact that numbers of cells secreting IL-13 in response to nickel were decreased in PBMC cultures from nonallergic subjects after addition of "7/4 cocktail" (possibly a cytotoxic effect, compare Fig. 1). Individual results for IL-5, IL-2, and LPT test protocols are shown in Figures 2-4. Regarding secretion of the "type 1" cytokine IFN-γ, no relevant differences were observed between Ni-ACD and Controls in any of the test protocols studied. IFN-γ ELISA without cytokine cocktails was closest to the selected significance level with P=0.062.
Table 1. In vitro test protocols with significant differences between Ni-ACD patients and Controls. ELISpot results are shown as numbers of secreting cells per 106 PBMC, ELISA results are in pg/106 PBMC. LPT results are values of the stimulation index (SI). Only results are shown, for which the difference between Ni-ACD and Controls was statistically significant (P≤0.05).
| In vitro test protocol |
Median result Controls |
Median result Ni-ACD |
Difference between Ni-ACD and Controls* |
P |
| IL-13 ELISpot with "7/4 cocktail" |
-7.5 |
135 |
105% |
<0.001 |
| IL-13 ELISpot without cocktail |
0 |
17.5 |
100% |
0.001 |
| IL-5 ELISA without cocktail |
0 |
14 |
100% |
0.001 |
| IL-5 ELISpot without cocktail |
0 |
10 |
100% |
0.007 |
| IL-5 ELISpot with "7/4 cocktail" |
10 |
140 |
93% |
<0.001 |
| IL-2 ELISpot with "7/12 cocktail" |
5 |
70 |
93% |
0.001 |
| IL-2 ELISpot no cocktail |
7.5 |
67.5 |
89% |
<0.001 |
| IL-5 ELISA with "7/4 cocktail" |
33 |
266 |
88% |
0.014 |
| LPT without cocktail |
1.4 |
5.5 |
74% |
<0.001 |
| LPT with "7/12 cocktail" |
1.2 |
2.3 |
48% |
0.001 |
| * The way of calculating the difference is described in Methods. |
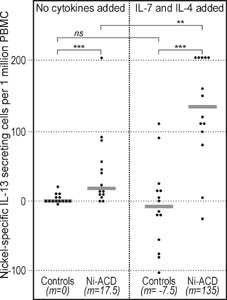
|
Figure 1. IL-13 secretion in response to nickel (ELISpot). The numbers of secreting cells were significantly higher among Ni-ACD patients than among Controls. Addition of the "7/4 cocktail" further increased the difference. The results were calculated as difference between the number of cells secreting cytokine in the presence of nickel minus the number of cells secreting the cytokine spontaneously in the absence of nickel (background) expressed per 106 PBMC. Symbols: **: P<0.01; ***: P<0.001; ns: not significant; m: median. Horizontal bars represent medians. |
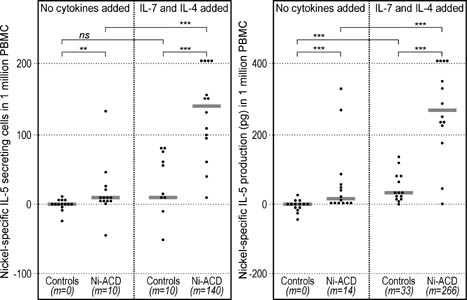
|
Figure 2. Analysis of nickel-specific IL-5 secretion by means of ELISpot (left) and ELISA (right). The numbers of cells secreting IL-5 (ELISpot) and the overall production of IL-5 (ELISA) in response to nickel were significantly higher among Ni-ACD patients than among Controls. Addition of the "7/4 cocktail" further increased the difference between groups. ELISpot results were calculated as in Fig. 1. ELISA results were presented as cytokine concentration in supernatants from cultures with nickel minus the concentration in cultures without nickel (spontaneous secretion), expressed per 106 PBMC. Symbols: **: P<0.01; ***: P<0.001; ns: not significant; m: median. Horizontal bars represent medians. |
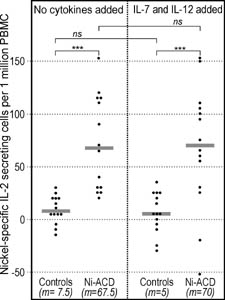
|
Figure 3. IL-2 secretion in response to nickel (ELISpot). The numbers of IL-2 secreting cells (ELISpot) were significantly higher among Ni-ACD patients than among Controls. Addition of the "7/12 cocktail" did not change the difference. The results were calculated as in Fig. 1. Symbols: ***: P<0.001; ns: not significant; m: median. Horizontal bars represent medians. |
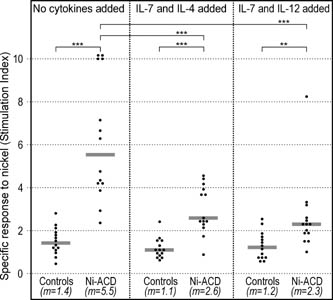
|
Figure 4. Lymphocyte proliferation test (LPT) in response to nickel. Results are shown as stimulation indexes (SI), i.e. radioactivity of cultures with nickel divided by radioactivity of cultures without the presence of nickel. Symbols: **: P<0.01; ***: P<0.001; m: median. Horizontal bars represent medians. |
Concordance between in vitro results and clinical diagnosis (Q2)
The strongest correlation with "golden standard" was observed in the case of LPT with the "7/4 cocktail", followed by IL-13 ELISpot with "7/4 cocktail" (Table 2). For all other protocols analysed, correlation coefficients r were below 0.5, with the lowest correlation observed for IFN-γ ELISpot.
Table 2. In vitro test protocols with highest (r > 0.5) correlation with clinical diagnosis (positive history and patch test).
| In vitro test protocol |
r |
P |
| LPT with "7/4 cocktail" |
0.739 |
<0.001 |
| IL-13 ELISpot with "7/4 cocktail" |
0.654 |
<0.001 |
| LPT without cocktail |
0.612 |
0.001 |
| IL-5 ELISpot with "7/4 cocktail" |
0.551 |
0.002 |
| IL-2 ELISpot with "7/12 cocktail" |
0.544 |
0.003 |
Concordance between non-radioactive test protocols and the LPT (Q3)
The strongest correlation was found between LPT and IL-2 ELISpot without cytokine cocktails, followed by IL-13 ELISpot with and without "7/4 cocktail" (Table 3). The correlation coefficients between LPT and IFN-γ ELISpot and ELISA assays were low (r ≤ 0.051) and not significant.
Table 3. Non-radioactive test protocols with highest (r > 0.5) correlation with the lymphocyte proliferation test (LPT).
| In vitro test protocol |
r |
P |
| IL-2 ELISpot without cocktail |
0.809 |
< 0.001 |
| IL-13 ELISpot without cocktail |
0.778 |
< 0.001 |
| IL-13 ELISpot with "7/4 cocktail" |
0.778 |
< 0.001 |
| IL-5 ELISA without cocktail |
0.669 |
< 0.001 |
| IL-2 ELISpot with "7/12 cocktail" |
0.587 |
0.001 |
DISCUSSION
Nickel is the most important contact allergen with sensitisation rates estimated at 17% among adults (23) and 8-10% among children (24, 25). In the present study, we compared clinical diagnosis of ACD with results of 15 in vitro test protocols, i.e. combinations of 3 in vitro assays (ELISpot, ELISA, LPT) with 3 various culture conditions (standard culture conditions, and 2 modifications with cytokine cocktails). Both cytokine cocktails used contained IL-7, which together with IL-4 or IL-12 enhances priming of naive cells (26, 27). IL-7 also counteracts apoptosis of allergen-specific naive and effector T lymphocytes (28).The development of "type 1" and "type 2" lymphocyte subpopulations was supported by adding IL-12 or IL-4, respectively (17). There is ongoing discussion, which lymphocyte subsets act as effector cells in ACD: T helper cells (CD4+), T cytotoxic cells (CD8+), other lymphocytes like NK or NKT cells, or maybe more than one subpopulation (29). Although this study was not specifically aimed at addressing this question, our results show that IL-5 and IL-13 production was the most prominent response to Ni, thus indicating on the prevailing "type 2" activation (possible effector cell phenotypes Th2, Tc2, NK2, and/or NKT2). The exact phenotype of these effector cells will be subject to further investigations.
Despite allergic contact dermatitis has traditionally been regarded as an IFN-γ-driven disease, we haven't observed any significant differences between Ni-ACD patients and Controls regarding IFN-γ production in response to nickel. Technically, this could be explained through e.g. a high spontaneous secretion of IFN-γ (possibly by NK cells), which could "obscure" the secretion by nickel-specific lymphocytes. However, there were previous hints on preferred "type 2" response to nickel of peripheral blood lymphocytes (30, 31), and also of Ni-specific lymphocytes from isolated eczematous skin (32, 33). The "type 2" cytokine pattern in response to Ni seems independent of atopic status of the sensitised person (34, 35). Moreover, this pattern seems not restricted to nickel only: Masjedi et al. found that allergen-specific IL-13 production discriminated best between cultures of PBMC from people with contact allergy to methylisothiazolinones and from controls (36). This encourages further studies of "type 2" test protocols also with other contact allergens.
The in vitro test protocol that correlated best with clinical diagnosis ("golden standard") was LPT done with "type 2" skewing (addition of "7/4 cocktail"). This observation was disappointing to some extent, as one of the aims of the study was to identify a non-radioactive alternative for the LPT. However, the "classical" LPT, i.e. done without enhancement with cytokine cocktails, was outperformed by IL-13 ELISpot with "7/4 cocktail". Finally, when looking for a non-radioactive alternative for the "traditional" LPT, the highest correlation was found between this method and IL-2 ELISpot (without cocktail). This finding fits well to the knowledge that memory cells secrete IL-2 to stimulate proliferation and differentiation after encounter with specific antigen (37). Relatively high correlations were also observed between LPT and IL-13 ELISpot and IL-5 ELISA, but again not in case of the "type 1" cytokine IFN-γ.
In concluding the above results, combining ELISpot assay with modified culture conditions may constitute a relevant progress in the detection of contact allergy to nickel in vitro. Among the test protocols analysed, IL-13 ELISpot in cultures with "7/4 cocktail" was most effective in detecting Ni-specific response and should be further evaluated regarding its possible use for in vitro diagnosis of contact allergy.
Acknowledgement
This study was funded by a grant from the European Commission (Marie Curie Individual Fellowship QLK4-CT-2002-51504 for Dr Radoslaw Spiewak).
REFERENCES
- Bruze M, Isaksson M, Edman B, Bjorkner B, Fregert S, Moller H. A study on expert reading of patch test reactions: inter-individual accordance. Contact Dermatitis 1995: 32: 331-337.
- van Strien G A, Korstanje M J. Site variations in patch test responses on the back. Contact Dermatitis 1994: 31: 95-96.
- Hindsen M, Bruze M, Christensen O B. Individual variation in nickel patch test reactivity. Am J Contact Dermat 1999: 10: 62-67.
- Spiewak R. Problems with interpreting the results of allergological patch tests: An analysis of test results in 196 patients with suspected contact dermatitis. Int Rev Allergol Clin Immunol 1997: 3 (Suppl 2): 36.
- Jonker M J, Bruynzeel D P. The outcome of an additional patch-test reading on days 6 or 7. Contact Dermatitis 2000: 42: 330-335.
- Aberer W. Die "falsch-positive" Epikutantest-Reaktion. Derm Beruf Umwelt 1988: 36: 13-16.
- Damian D L, Barnetson R S, Halliday G M. Effects of low-dose ultraviolet radiation on in vivo human cutaneous recall responses. Australas J Dermatol 2001: 42: 161-167.
- Green C. The effect of topically applied corticosteroid on irritant and allergic patch test reactions. Contact Dermatitis 1996: 35: 331-333.
- Anveden I, Lindberg M, Andersen K E, Bruze M, Isaksson M, Liden C, Sommerlund M, Wahlberg J E, Wilkinson J D, Willis C M. Oral prednisone suppresses allergic but not irritant patch test reactions in individuals hypersensitive to nickel. Contact Dermatitis 2004: 50: 298-303.
- Mitchell J C. The angry back syndrome: eczema creates eczema. Contact Dermatitis 1975: 1: 193-194.
- Bruynzeel D P, van Ketel W G, von Blomberg-van der Flier M, Scheper R J. Angry back or the excited skin syndrome. A prospective study. J Am Acad Dermatol 1983: 8: 392-397.
- Rocklin R E, Meyers O L, David J R. An in vitro assay for cellular hypersensitivity in man. J Immunol 1970: 104: 95-102.
- Geczy A F, Baumgarten A. Lymphocyte transformation in contact sensitivity. Immunology 1970: 19: 189-203.
- Macleod T M, Hutchinson F, Raffle E J. The uptake of labelled thymidine by leucocytes of nickel sensitive patients. Br J Dermatol 1970: 82: 487-492.
- von Blomberg-van der Flier M, van der Burg C K, Pos O, van de Plassche-Boers E M, Bruynzeel D P, Garotta G, Scheper R J. In vitro studies in nickel allergy: diagnostic value of a dual parameter analysis. J Invest Dermatol 1987: 88: 362-368.
- Hallab N J, Mikecz K, Jacobs J J. A triple assay technique for the evaluation of metal-induced, delayed-type hypersensitivity responses in patients with or receiving total joint arthroplasty. J Biomed Mater Res 2000: 53: 480-489.
- Rustemeyer T, von Blomberg B M, van Hoogstraten I M, Bruynzeel D P, Scheper R J. Analysis of effector and regulatory immune reactivity to nickel. Clin Exp Allergy 2004: 34: 1458-1466.
- Jakobson E, Masjedi K, Ahlborg N, Lundeberg L, Karlberg A T, Scheynius A. Cytokine production in nickel-sensitized individuals analysed with enzyme-linked immunospot assay: possible implication for diagnosis. Br J Dermatol 2002: 147: 442-449.
- Lindemann M, Bohmer J, Zabel M, Grosse-Wilde H. ELISpot: a new tool for the detection of nickel sensitization. Clin Exp Allergy 2003: 33: 992-998.
- Minang J T, Ahlborg N, Troye-Blomberg M. A simplified ELISpot assay protocol used for detection of human interleukin-4, interleukin-13 and interferon-γ production in response to the contact allergen nickel. Exogenous Dermatology 2003: 2: 306-313.
- Moed H, Stoof T J, Boorsma D M, von Blomberg B M E, Gibbs S, Bruynzeel D P, Scheper R J, Rustemeyer T. Identification of anti-inflammatory drugs according to their capacity to suppress type-1 and type-2 T cell profiles. Clin Exp Allergy 2004: 34: 1868-1875.
- Jennes W, Kestens L, Nixon D F, Shacklett B L. Enhanced ELISPOT detection of antigen-specific T cell responses from cryopreserved specimens with addition of both IL-7 and IL-15 - the Amplispot assay. J Immunol Methods 2002: 270: 99-108.
- Uter W, Hegewald J, Aberer W, Ayala F, Bircher AJ, Brasch J, Coenraads PJ, Schuttelaar ML, Elsner P, Fartasch M, Mahler V, Belloni Fortina A, Frosch PJ, Fuchs T, Johansen JD, Menne T, Jolanki R, Krecisz B, Kiec-Swierczynska M, Larese F, Orton D, Peserico A, Rantanen T, Schnuch A. The European standard series in 9 European countries, 2002/2003 - first results of the European Surveillance System on Contact Allergies. Contact Dermatitis 2005: 53: 136-145.
- Spiewak R. Allergische Kontaktdermatitis im Kindesalter. Eine Übersicht und Meta-Analyse. Allergologie 2002: 25: 374-381.
- Heine G, Schnuch A, Uter W, Worm M. Frequency of contact allergy in German children and adolescents patch tested between 1995 and 2002: results from the Information Network of Departments of Dermatology and the German Contact Dermatitis Research Group. Contact Dermatitis 2004: 51: 111-117.
- Rustemeyer T, de Ligter S, von Blomberg B M, Frosch P J, Scheper R J. Human T lymphocyte priming in vitro by haptenated autologous dendritic cells. Clin Exp Immunol 1999: 117: 209-216.
- Vakkila J, Aysto S, Saarinen-Pihkala U M, Sariola H. Naive CD4+ T cells can be sensitized with IL-7. Scand J Immunol 2001: 54: 501-505.
- Park J H, Yu Q, Erman B, Appelbaum J S, Montoya-Durango D, Grimes H L, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 2004: 21: 289-302.
- Kimber I, Dearman R J. Allergic contact dermatitis: the cellular effectors. Contact Dermatitis 2002: 46: 1-5.
- Borg L, Christensen J M, Kristiansen J, Nielsen N H, Menne T, Poulsen L K. Nickel-induced cytokine production from mononuclear cells in nickel-sensitive individuals and Controls. Cytokine profiles in nickel-sensitive individuals with nickel allergy-related hand eczema before and after nickel challenge. Arch Dermatol Res 2000: 292: 285-291.
- Minang J T, Troye-Blomberg M, Lundeberg L, Ahlborg N. Nickel elicits concomitant and correlated in vitro production of Th1-, Th2-type and regulatory cytokines in subjects with contact allergy to nickel. Scand J Immunol 2005: 62: 289-296.
- Probst P, Kuntzlin D, Fleischer B. TH2-type infiltrating T cells in nickel-induced contact dermatitis. Cell Immunol 1995: 165: 134-140.
- Büdinger L, Neuser N, Totzke U, Merk H F, Hertl M. Preferential usage of TCR-Vβ17 by peripheral and cutaneous T cells in nickel-induced contact dermatitis. J Immunol 2001: 167: 6038-6044.
- Szepietowski J C, McKenzie R C, Keohane S G, Aldridge R D, Hunter J A. Atopic and non-atopic individuals react to nickel challenge in a similar way. A study of the cytokine profile in nickel-induced contact dermatitis. Br J Dermatol 1997: 137: 195-200.
- Spiewak R. Atopy and contact hypersensitivity: a reassessment of the relationship using objective measures. Ann Allergy Asthma Immunol 2005: 95: 61-65.
- Masjedi K, Ahlborg N, Gruvberger B, Bruze M, Karlberg A T. Methylisothiazolinones elicit increased production of both T helper (Th)1- and Th2-like cytokines by peripheral blood mononuclear cells from contact allergic individuals. Br J Dermatol 2003: 149: 1172-1182.
- Lindemann MJ, Benczik M, Gaffen SL. Anti-apoptotic signaling by the interleukin-2 receptor reveals a function for cytoplasmic tyrosine residues within the common gamma (gamma c) receptor subunit. J Biol Chem 2003: 278: 10239-10249.
© Radoslaw Spiewak. (contact).
This page is part of the www.RadoslawSpiewak.net website.
Document created: 3 November 2006, last updated: 25 November 2021.



