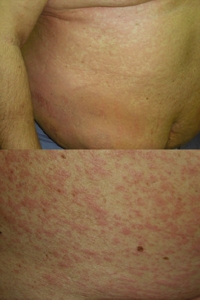
Fig. 1. Heparin-induced cutaneous adverse drug reactions: generalized urticaria (top) and the subsequent maculopapular exanthema (bottom).
|
Introduction: We present a case of generalized urticaria followed by maculopapular rash in response to heparin, accompanied by a conspicuous rise in peripheral eosinophilia and serum IgE level that subsided after clearance of the skin rash. Key words: cutaneous adverse drug reactions; drug allergy; heparins; hypereosinophilia; IgE. |
A 47-year-old man was admitted for inpatient treatment of acute febrile deep venous thrombosis of the right leg. His medical history included recurrent venous thrombosis, patent foramen ovale, pulmonary embolism, impaired cerebral perfusion, inferior vena cava filter implant, obesity, arterial hypertension, and ventricular arrhythmia. The patient had been treated for 21 years with anticoagulants (mainly acenocoumarol, and occasionally heparins). Current treatment included immobilization of the affected leg, and continuous infusions of unfractionated heparin (Heparinum natricum 70 000 IU daily) for 10 days. Accessory therapy included cefotaxime, metronidazole, paracetamol, mexiletine, acebutolol, indapamide, and potassium. On day 11, unfractionated heparin was replaced with the low molecular weight heparin (LMWH) enoxaparin sodium (Clexane®) (120 mg, twice-daily subcutaneous) and acenocoumarol (4 mg, twice-daily, oral). After two further days of treatment, the patient was released in good condition with instructions to continue with Clexane®, acenocoumarol, and his cardiac drugs.
Approximately 40 hr after the first injection of Clexane®, the patient was re-admitted because of itching urticarial lesions on an erythematous background appearing locally around injection sites of the drug that appeared within minutes after each administration. Clexane® was immediately replaced with another LMWH, nadroparin calcium (Fraxiparine®), and acenocoumarol and cardiac drugs were continued, because of clinical need. Fraxiparine® had to be discontinued after two injections, because the rash continued to generalize, evolving gradually into pruritic exanthema, initially rubella-like but subsequently maculopapular. On the following day, the patient’s whole body was involved (Fig. 1) but no wheals were seen at this stage. Apart from abnormalities that could be attributed to the patient’s thrombosis and treatment, laboratory tests revealed marked hypereosinophilia (1.748 G/l, 18.4% of leukocytes) and a very high total IgE level (2781 IU/ml). Complete resolution of the skin rash was achieved after 8 days of prednisolone (50 mg, intravenous, twice-daily), cetirizine (10 mg, oral, twice-daily), and hydrocortisone 1% ointment.
|
Fig. 1. Heparin-induced cutaneous adverse drug reactions: generalized urticaria (top) and the subsequent maculopapular exanthema (bottom). |
Four months later, the patient underwent testing. His blood eosinophilia and the total IgE count had decreased to 0.384 G/l (6.4%) and 694 IU/ml, respectively. All three heparins used by the patient – Heparinum natricum, Clexane®, and Fraxiparine® – were tested. The results of prick tests (15 and 30 min) and patch tests (D3/D4) with undiluted drugs remained negative. Intracutaneous test results were positive with all three heparins at the original concentrations and 1:10 dilutions: After 30 min, the wheal diameters were 6–8 mm, with erythema 22–25 mm; the reactions fully resolved within 8 hr. Control tests in five other patients receiving anticoagulant therapy gave negative results.
During the episode of heparin hypersensitivity, the patient showed a peculiar biphasic reaction that initially emerged as urticaria, and later evolved as maculopapular exanthema. The positive intracutaneous test result seems to be consistent with the initial urticaria. Patch test results remained negative, which might either indicate the absence of delayed-type hypersensitivity, or reflect the lack of penetration of the drugs (4500–30 000 MW) into intact epidermis. Most conspicuous was the significantly increased eosinophilia (18.4%), which subsided after resolution of the skin rash. Eosinophilia is considered to be a frequent phenomenon during heparin therapy (1). In the present case, however, it seems to be connected to the cutaneous adverse reaction, as the patient’s eosinophil counts recorded on several preceding occasions (including during previous courses of heparin therapy) ranged from 5.1% to 6.0%, and returned to 6.4% after the episode. Also, eosinophilic infiltrates observed in skin lesions of patients with heparin hypersensitivity (2–5) may indicate a more active role of eosinophils. We were able to exclude the drug rash with eosinophilia and systemic symptoms (DRESS) syndrome in our patient, as his body temperature did not exceed 36.7◦C, there was no lymphadenopathy, and there were no signs of hepatic, renal, pulmonary or pancreatic pathology on physical examination, diagnostic imaging, and laboratory testing. Up to now, blood hypereosinophilila (with a normal IgE level) has been reported in only one case of a systemic adverse reaction to heparin (6). The increased total IgE seen in our patient might have resulted from increased secretion of interleukin-4, and the eosinophilia from interleukin-5 overproduction by activated ‘type 2’ lymphocytes (Th2, Tc2 or NKT2 cells).
© Radoslaw Spiewak (contact).
This page is part of the www.RadoslawSpiewak.net website.
Document created: 28 December 2010, last updated: 1 September 2011.